An Introduction to rhBMP-2

rhBMP-2
Introduction
rhBMP-2
Structure
rhBMP-2
Application
rhBMP-2
Researches
rhBMP-2
Product
Contact Us


rhBMP-2 delivery: How surface interactions modulate release in vitro and in vivo
Biomaterial scaffolds have been extensively used to deliver growth factors to induce new bone formation. The pharmacokinetics of growth factor delivery has been a critical regulator of their clinical success. This review will focus on the surface interactions that control the non-covalent incorporation of growth factors into scaffolds and the mechanisms that control growth factor release from clinically relevant biomaterials. We will focus on the delivery of recombinant human bone morphogenetic protein-2 from materials currently used in the clinical practice, but also suggest how general mechanisms that control growth factor incorporation and release delineated with this growth factor could extend to other systems. A better understanding of the changing mechanisms that control growth factor release during the different stages of preclinical development could instruct the development of future scaffolds for currently untreatable injuries and diseases.
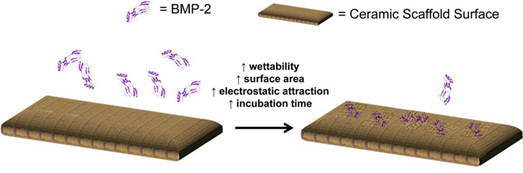
Schematic representation of BMP-2 adsorption to ceramic scaffolds with the properties that generally have increased the mass of BMP-2 adsorbed to its surface.
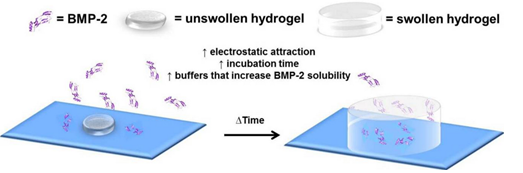
Schematic representation of BMP-2 incorporation into a dried polymer network that swells to form a hydrogel and properties that generally increase the mass of BMP-2 incorporated into the hydrogel network.
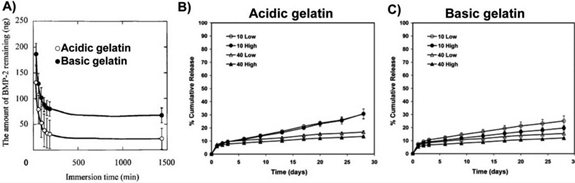
Results of studies that compared rhBMP-2 release from acidic and basic gelatin hydrogels in vitro. A) rhBMP-2 release was faster from acidic gelatin hydrogel disks compared to basic gelatin hydrogel disks in PBS with permission. rhBMP-2 release was also faster from acidic gelatin hydrogel microparticles (B) crosslinked with 10 or 40mM glutaraldehyde at low or high rhBMP-2 doses compared to basic gelatin hydrogel microparticles (C) with permission.
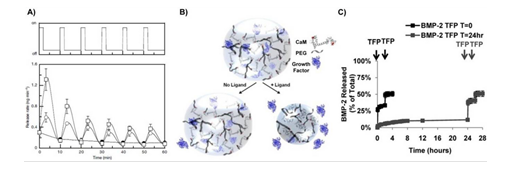
Influence of convective fluid flow on growth factor release in vitro. A) Mechanical strain (top) triggered the release of VEGF from alginate hydrogels over the course of 6 loading cycles (bottom) with permission. B) Schematic representation of dynamic hydrogels in which specific biochemical ligands induced protein-conformational changes, hydrogel volume decreases, and growth factor release with permission. C) Temporal control over BMP-2 release from dynamic hydrogel networks was controlled by varying the timing that hydrogels underwent trifluoperazine (TFP)-induced volume decreases with permission.

Clonal Isolation of Muscle-Derived Cells Capable of Enhancing Muscle Regeneration and Bone Healing
Several recent studies suggest the isolation of stem cells in skeletal muscle, but the functional properties of these muscle-derived stem cells is still unclear. In the present study, we report the purification of muscle-derived stem cells from themdx mouse, an animal model for Duchenne muscular dystrophy. We show that enrichment of desmin+ cells using the preplate technique from mouse primary muscle cell culture also enriches a cell population expressing CD34 and Bcl-2. The CD34+ cells and Bcl-2+ cells were found to reside within the basal lamina, where satellite cells are normally found. Clonal isolation and characterization from this CD34+Bcl-2+enriched population yielded a putative muscle-derived stem cell, mc13, that is capable of differentiating into both myogenic and osteogenic lineage in vitro and in vivo. The mc13 cells are c-kit and CD45 negative and express: desmin, c-met and MNF, three markers expressed in early myogenic progenitors; Flk-1, a mouse homologue of KDR recently identified in humans as a key marker in hematopoietic cells with stem cell-like characteristics; and Sca-1, a marker for both skeletal muscle and hematopoietic stem cells. Intramuscular, and more importantly, intravenous injection of mc13 cells result in muscle regeneration and partial restoration of dystrophin in mdx mice. Transplantation of mc13 cells engineered to secrete osteogenic protein differentiate in osteogenic lineage and accelerate healing of a skull defect in SCID mice. Taken together, these results suggest the isolation of a population of muscle-derived stem cells capable of improving both muscle regeneration and bone healing.
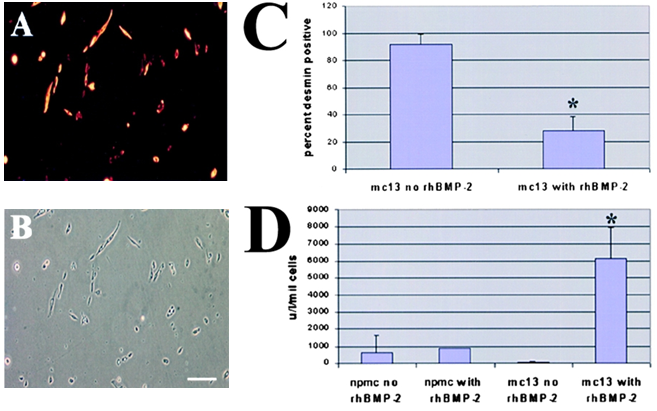
The effect of rhBMP-2 on the expression of desmin and alkaline phosphatase by the mc13 cells. Desmin staining of freshly isolated mc13 clones (A) showed that all of these cells express desmin. The phase-contrast view (B) is shown to demonstrate that a high percentage of the cells were desmin positive. Mc13 cells were incubated in growth media containing 200 ng/ml rhBMP-2 for 6 d. Cells were then stained for desmin expression, and percent desmin positive cells calculated by visualization with immunofluorescence. As a control, mc13 cells were grown in parallel without addition of rhBMP-2. When grown without rhBMP-2, mc13 cells remained uniformly (90每100%) desmin positive (C). With exposure to rhBMP-2, there is a significant decrease (*P < 0.05) in relative number of desmin positive cells (30每40%) within 6 d (C). The npmc and mc13 cells were also analyzed for expression of alkaline phosphatase after 6 d of growth with and without exposure to 200 ng/ml rhBMP-2. With rhBMP-2 stimulation, mc13 cells show a >600-fold increase in alkaline phosphatase activity than nonstimulated cells (D). The npmc show only minimal alkaline phosphatase activity with or without rhBMP-2 (D). *, Indicates a significant difference using the t test (P < 0.05). Bar, 100 µm.
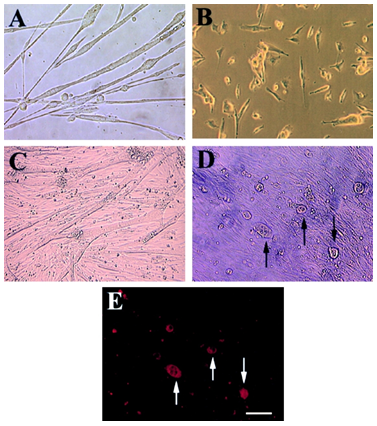
Morphologic change and expression of osteocalcin by mc13 cells with exposure to rhBMP-2. Mc13 cells were incubated in growth media without rhBMP-2 for 6 d. When cells became >50% confluent, they began to fuse and form multinucleated myotubes (A). When mc13 cells were incubated in growth media containing 200 ng/ml rhBMP-2, cells remained mononucleated and did not fuse (B). When cells reached >90% confluency without rhBMP-2, almost all the cells fused to form myotubes (C). With rhBMP-2, when cells reached >90% confluency, round, hypertrophic cells began to appear in the culture (D, arrows). These round, hypertrophic cells were highly positive for osteocalcin expression (E, arrows). Bar, 50 µm.

Bone morphogenetic protein-2: a potential regulator in scleral remodeling
Bone morphogenetic protein 2 (BMP-2) is a member of the main subgroup of bone morphogenetic proteins within the transforming growth factor-β superfamily. BMP-2 is involved in numerous cellular functions including development, cell proliferation, apoptosis, and extracellular matrix synthesis. We examined BMP-2 expression in human scleral fibroblasts (HSF) and assessed the effects of recombinant human BMP-2 (rhBMP-2) on HSF proliferation, matrix metalloproteinase-2 (MMP-2), and tissue inhibitor of metalloproteinase-2 (TIMP-2).
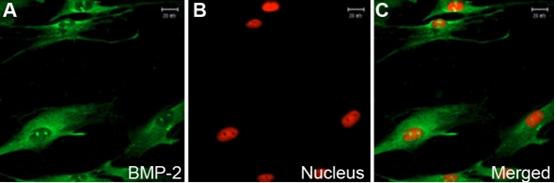
Distribution of BMP-2 in human sclera fibroblasts in vitro using indirect immunofluorescence. FITC marked the secondary antibody (green; A), and PI dyed the nucleus (red; B). The first (A) and second images (B) are combined to form the third image shown (C). BMP-2 is localized in the cytoplasm and weakly in the nucleus of HSF (A-C). Magnification: 400x.
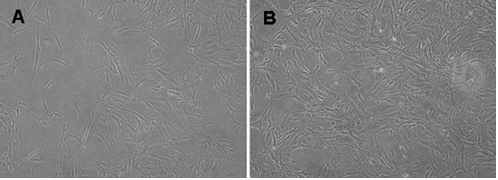
The changed pattern of human sclera fibroblasts incubated with rhBMP-2. The HSFs showed only a sparse and vortex pattern before incubation (A) whereas incubation with 100 ng/ml rhBMP-2 changed the HSFs into the intensive and polygonal cells (B). Inverted phase contrast microscope, original magnification 100 x.
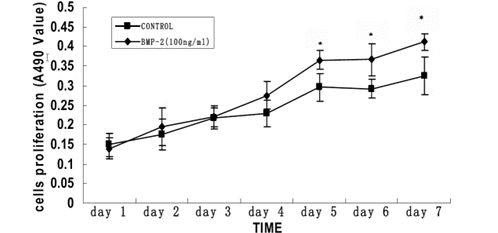
Time-dependent effects of rhBMP-2 on the cell proliferation of primary cultured human sclera fibroblasts. Cell proliferation was determined by MTT assay. The optical density value at A490 and the mean㊣SD is provided for each time point (n=8). The cells were incubated with no rhBMP-2 or 100ng/ml rhBMP-2 for seven days. A significant difference was seen in cell proliferation between 100 ng/ml rhBMP-2 treatment and control after four days. The asterisk indicates that P<0.05 when compared with the control without rhBMP-2.
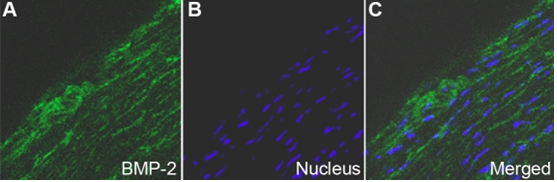
Distribution of BMP-2 in human sclera by indirect immunofluorescence. FITC marked the secondary antibody (green; A) and Hoechst33342 dyed the nucleus (blue; B). The first (A) and second images (B) are combined to form the third image shown (C). BMP-2 is localized in the cytoplasm of HSF and extracellular matrices(A-C). Magnification: 400x.
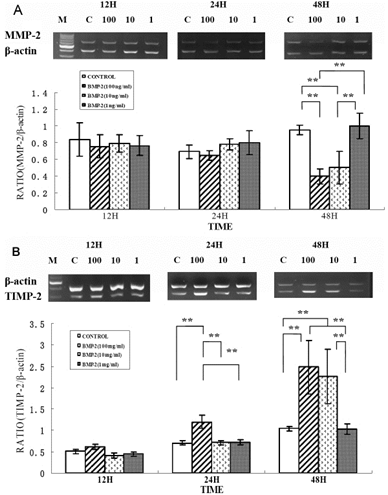
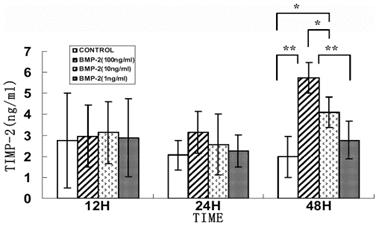
Effect of rhBMP-2 on mRNA expression levels of MMP-2 and TIMP-2 in human sclera fibroblasts. Ethidium-bromide agarose gels indicated the level of 汕-actin message relative toMMP-2 (A) and TIMP-2 (B) levels from total RNA. Bar graphs revealed changes in mRNA expression where values were normalized to beta-actin values and expression was state as a ratio of optical dencity. The double asterisk means that the semi-quantitative RT-PCR revealed significant changes for MMP-2 and TIMP-2 with varying concentration of rhBMP-2.
Distribution of BMP-2 in human sclera by indirect immunofluorescence. FITC marked the secondary antibody (green; A) and Hoechst33342 dyed the nucleus (blue; B). The first (A) and second images (B) are combined to form the third image shown (C). BMP-2 is localized in the cytoplasm of HSF and extracellular matrices(A-C). Magnification: 400x.
©2013 BiologicsCorp, All right reserved.
Contact Us


